Answer:
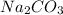 or Sodium carbonate.
or Sodium carbonate.
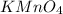 or Potassium permanganate
or Potassium permanganate
Sodium ion carries 1 + charge whereas carbonate ion carries overall charge of -2. So for every carbonate ion, two sodium ions combine to balance the charges and thus form a stable compound,
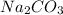
Potassium ion carries 1 + charge where as permanganate ion carries overall charge of -1. So for every permanganate ion, one potassium ion combines to balance the charges and thus form a stable compound,
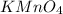
The predicted formulas are shown in the attached image.