Answer: A) become weaker.
Explanation:
Melting is the process in which solid phase is converted to liquid phase.
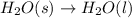
Solid state : In this state, the molecules are arranged in regular and repeating pattern. The molecules are closely packed as they have strong forces of interaction. The particles are fixed and vibrate in place but they can not move from one place to another.
Liquid state : In this state, the molecules are present in random and irregular pattern. The molecules are closely packed but they can move from one place to another. as they have weak forces of attraction.
Thus as solid gets converted to liquid, the forces of attraction become weaker.