Answer: Option (C) is the correct answer.
Step-by-step explanation:
According to Lewis, a base is a substance or specie which is able to donate electrons.
For example,
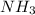 is a lewis base because nitrogen has a lone pair of electron.
is a lewis base because nitrogen has a lone pair of electron.
Therefore, when ammonia is dissolved in HCl it will accept the hydrogen atom as nitrogen gave electron to the hydrogen atom. As a result, hydrogen bonds with nitrogen atom.
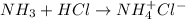
Thus, we can conclude that it donates electrons in solution best describes a Lewis base.