Answer:
The pressure will double
Step-by-step explanation:
Hello,
In this case, by considering the Boyle's law which allows us to understand the pressure-volume behavior of a gas in an inversely proportional relationship:
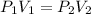
It is said that the volume gets halved, thus:
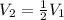
In such a way the pressure will result in:
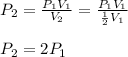
Therefore, the pressure will double.
Best regards.