Answer:
The volume upto which a gas can be compressed at 5.2 atm pressure is 33.65 L.
Step-by-step explanation:
Under constant temperature:
Initial volume of the gas
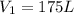
Initial pressure of the gas =
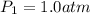
Final volume of the gas
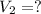
Final pressure of the gas =
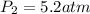
Applying Boyle's law:
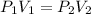 (constant temperature)
(constant temperature)
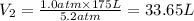
The volume upto which a gas can be compressed at 5.2 atm pressure is 33.65 L.