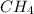Answer: The empirical formula is
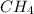
Step-by-step explanation:
Empirical formula is defined as the formula in which atoms in a compound are present in simplest whole number ratios.
For the given options:
Option A:
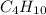
As, the atoms are not in the smallest whole number ratio, it is not the empirical formula. The empirical formula of this will be
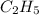
Option B:
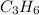
As, the atoms are not in the smallest whole number ratio, it is not the empirical formula. The empirical formula of this will be
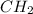
Option C:
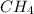
As, the atoms are in the smallest whole number ratio, it is the empirical formula.
Option D:
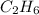
As, the atoms are not in the smallest whole number ratio, it is not the empirical formula. The empirical formula of this will be
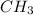
Hence, the empirical formula is