Answer : The correct option is, (B) The
![[CO]](https://img.qammunity.org/2018/formulas/chemistry/high-school/srofwh05rsjlp5po2mzq6fhs1r18jh6jzs.png) decreases and
decreases and
![[CO_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/hrae46iljxaftz47d23do125fri3zzybtb.png) increases.
increases.
Explanation :
According to the Le-Chatelier's principle, if any changes occurs in the variables of the reaction then the equilibrium will be shift in that direction where the effect is minimum.
When the concentration of the reactant increases then the equilibrium will be shift in the forward direction and when the concentration of the reactant decreases then the equilibrium will be shift in the backward or reverse direction.
As per question, when a closed container is initially filled with
 and CO then the concentration of reactants
and CO then the concentration of reactants
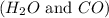 would decreases and concentration of
would decreases and concentration of
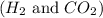 would increases.
would increases.
Hence, the correct option is, (B) The
![[CO]](https://img.qammunity.org/2018/formulas/chemistry/high-school/srofwh05rsjlp5po2mzq6fhs1r18jh6jzs.png) decreases and
decreases and
![[CO_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/hrae46iljxaftz47d23do125fri3zzybtb.png) increases.
increases.