Step-by-step explanation:
Initial temperature of water = 24.85°C
Final temperature of water = 35.65°C
Mass of water = 1000 g
The specific heat of water ,c = 4.184 J/g °C.
The heat capacity of the calorimeter
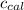 = 695 J/ °C
= 695 J/ °C
Change in temperature
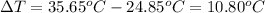
Heat absorbed by the water =
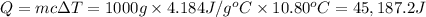
Heat absorbed by the calorimeter =
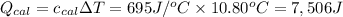
Total heat combustion =
Heat absorbed by the water + Heat absorbed by the calorirmeter
=(-45,187.2 J)+ (-7,506 J) = -52,693.2 J
1.11 grams of methane gives off 52,693.2 J.
Number of moles methane burned in an experiment =
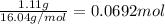
Experimental molar heat of combustion =
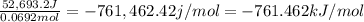
Negative sign heat is released during burning of methane.
The experimental data might differ from the theoretical value because while performing an experiment there are certain factors which affects the the observations and experimental procedure which results in differ value experimental value from theoretical value.
The percentage error in the experiment:
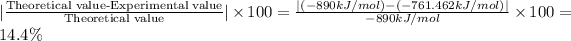
The percent error for the experiment is 14.4 %.