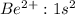Answer: The electron structure of
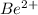 ion is
ion is
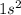
Step-by-step explanation: Beryllium is the 4th element of the periodic table.
The electronic configuration of Be-element in its ground state is:
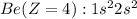
For the Beryllium element to have +2 charge, two electrons must be removed from the electronic configuration. So, the electronic configuration for the
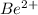 ion is:
ion is: