To calculate the limiting reactant, we need to compair the number of moles we have of each and see which have less, considering the stoichiometry.
So, first we need to convert the mass of Mg to number of moles, which we can do by using its molar mass:
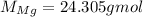
Thus, the number of moles of Mg is:
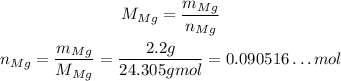
To calculate the number of moles of O₂, since it is in STP, the volume is directly proportional to the number of moles. The given value for STP volyme is 22.4L, so 1 mol of O₂ will have 22.4L, thus, using rule of three, we have:
n --- 4.5L
1mol --- 22.4L
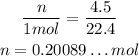
Now, for each 2 mols of Mg, 1 mol of O₂ will react. So, if all 0.20089...mol of O₂ react, we would need double amount of Mg, that is, we would need 0.40178...mol of Mg.
However, we have only 0.090516...mol of Mg, so we don't have enough Mg to react with all the O₂ we have.
This means that the Mg is the limiting reactant.