1) Determine the number of subatomic particles.
A subatomic particle is smaller than an atom. Protons, neutrons, and electrons are subatomic particles.
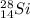
Mass number: 28
This number is the number of protons plus neutrons in the nucleus.
Atomic number: 14
This is the number of protons in the nucleus.
Neutrons = mass number - atomic number
Neutrons = 28 - 14 = 14.
Electrons. = atomic number - charge
Electrtons = 14 - 0 = 14
This species of Silicon has 14 protons, 14 neutrons, and 14 electrons.