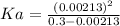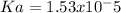Answer:
Step-by-step explanation:
Since it is a weak acid it is necessary to write the balance. Where the unknown acid will be HA.
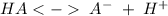 .
.
Then with the equilibrium we can write the ICE table (figure 1).
Where we obtain the equation:
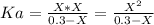
Now, we have to find "X". To do this we can use the pH equation and the pH value:
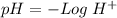
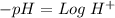
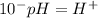
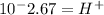
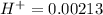
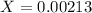
Then we have to plug the values in the Ka equation: