Answer:
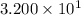
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number
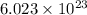 of particles.
of particles.
1 mole of
 weighs= 32.0 g
weighs= 32.0 g
Significant digits are each of the digits of a number that are used to express a number to the required degree of accuracy, starting from the first non-zero digit with exponents raised to the power of 10.
The number is written in scientific notation as
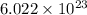 contains 4 significant digits.
contains 4 significant digits.
Thus 32 can be expressed as
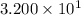 contains 4 significant digits.
contains 4 significant digits.