Answer: Option (C) is the correct answer.
Step-by-step explanation:
Elements of group 4A include carbon, silicon, germanium, tin and lead.
Also, it is known that valence shell is the shell which is present in the outermost side of an element.
Hence, the electrons present in this outermost shell are known as valence electrons.
For example, atomic number of carbon is 6 and its electronic configuration is
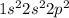 .
.
This means that there are 4 electrons present in the outermost shell.
Thus, we can conclude that group 4A (or 14) elements have 4 electrons in their valence shell.