Answer: Volume occupied by oxygen gas at STP is 16.78 liters.
Step-by-step explanation:
At STP, value of pressure ,P = 1atm
At STP, value of Temperature ,T = 273 K
Number moles of oxygen gas ,n= 0.75 moles
According to ideal gas equation :
PV=nRT, R = Universal Gas constant = 0.0820 L atm/mol K
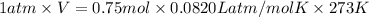
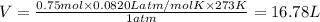
Volume occupied by oxygen gas at STP is 16.78 liters.