C₄H₁₀ +
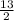
O₂ → 4CO₂ + 5H₂O
If mass of butane = 7.63 g
and mole = mass ÷ molar mass
then mole of butane = 7.63 g ÷ ((12 × 4) + (1 × 10))
= 7.63 g ÷ 58
= 0.1316 mol
Mole Ratio of C₄H₁₀ : H₂O is 1 : 5
∴ if mole of butane = 0.1316 mol
then mole of water = (0.1316 mol × 5 )
= 0.658 mol
Since mass = moles × molar mass
then mass of water produced = 0.658 mol × ((1 × 2) + (16 × 1))
= 11.844 g