Answer:
The heat transferred is 157 kJ.
Explanation:
Given : If 75 g of liquid water ( C = 4.18 j/g C ) in a calorimeter changes temperature from 25 C to 75 C.
To find: How much heat was transferred ?
Solution :
Using the formula,
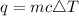
Where,
q - heat absorbed
c - the specific heat of the substance, in your case of water
ΔT - the change in temperature, as the difference between the final temperature and the initial temperature.
We have given,
c=4.18 j/g C
m=75 g
ΔT= 75 C-25 C=50 C
Substitute the values in the formula,
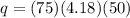
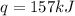
Therefore, The heat transferred is 157 kJ.