Answer: The correct answer is True.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces the less reactive element from its chemical reaction. General equation for this reaction follows:
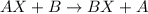
B is more reactive element than A.
Redox reaction is defined as the reaction in which oxidation and reduction reactions occur simultaneously.
Oxidation reaction is defined as the chemical reaction where a substance looses its electrons. The oxidation state of the substance gets increased and the substance gets oxidized.
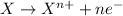
Reduction reaction is defined as the chemical reaction where a substance gains electrons. The oxidation state of the substance gets reduced and the substance gets reduced.
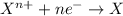
Examples of single displacement and redox reactions:
- Single displacement reactions:
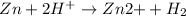
Here, zinc is loosing 2 electrons so it is undergoing oxidation reaction and hydrogen ion is gaining 2 electrons. So, it is undergoing reduction reaction. Thus, it is considered as a redox reaction.
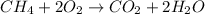
Here, oxidation state of carbon is increasing from -4 to +4, so it is undergoing oxidation reaction and oxidation state of oxygen is decreasing from 0 to -2, so it is undergoing reduction reaction. The above reaction is not a single displacement reaction.
From above information, it is clearly seen that all the single displacement reaction are redox reaction but reverse is not correct.
Hence, the correct answer is True.