Answer 1) Option D)
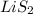
Explanation : The valency of Lithium is 1, which means lithium can lose one electron from its outermost valence shell and gain a positive charge on itself. It is combined with two moles of sulfur which is over satisfying the valency of lithium and is not abiding the rule of forming a molecular orbital.
Hence,
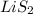 is the answer for not being the correct ionic formula.
is the answer for not being the correct ionic formula.
Answer 3) Option 4) AlN
Explanation : When aluminum is combining with nitrogen, aluminum has 3 valence electrons in its outermost shell. When nitrogen needs 3 electrons to form a stable bond with aluminum they share their electrons and achieve a stable state compound which is AlN. These 3 moles of Al needs 3 moles of N so, the chemical formula becomes AlN.
Answer 4)
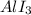
Explanation : The formula of a compound formed between iodine (I) and aluminum (Al)will be
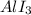 . Here, one mole of Al is combined with 3 moles of Iodine as iodine has the valency as 1 and Aluminum has 3 as its valency. So, it would require 3 moles of iodine to combine with one mole of Al and form a stable compound.
. Here, one mole of Al is combined with 3 moles of Iodine as iodine has the valency as 1 and Aluminum has 3 as its valency. So, it would require 3 moles of iodine to combine with one mole of Al and form a stable compound.
Answer 5) Option C)
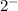
Explanation :
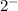 It is seen that all Group 16 elements which are nonmetals gain two electrons to form an ion with a
It is seen that all Group 16 elements which are nonmetals gain two electrons to form an ion with a
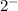 charge. So, when oxygen forms oxide ion the charge of the ion is
charge. So, when oxygen forms oxide ion the charge of the ion is
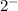 . As, the atom of oxide ion gains two electrons to form complete octet.
. As, the atom of oxide ion gains two electrons to form complete octet.
Answer 6) Option C) LiCl.
Explanation : A formula unit in chemistry is defined as the empirical formula of any ionic or covalent network solid compound which is used as an independent entity for stoichiometric calculations. It is assumed to be the lowest whole number ratio of ions represented in an ionic compound. Lithium has one electron excess and Chlorine needs one electron attain the octet state. So, Li gives an electron to Cl and forms a compound LiCl.
Answer 7) Option B) 3,1
Explanation :The complete formula unit is
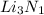 . Lithium has one valence electron in its outermost valence shell and Nitrogen needs 3 electrons to be an octet. So, 3 moles of Lithium are needed to combine with one mole of Nitrogen and form
. Lithium has one valence electron in its outermost valence shell and Nitrogen needs 3 electrons to be an octet. So, 3 moles of Lithium are needed to combine with one mole of Nitrogen and form
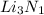 which can be also written as
which can be also written as
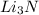 .
.
Answer 8)
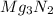
Explanation : The formula unit when magnesium (Mg) and Nitrogen (N) bond is formed it will be
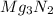 . As magnesium has the valency as 2 and nitrogen has 3 as its valency. Nitrogen is converted into nitride. As 3 moles of Mg will have 6 electrons in all and 2 moles of nitrogen will have 6 electrons. So, the formula unit will be
. As magnesium has the valency as 2 and nitrogen has 3 as its valency. Nitrogen is converted into nitride. As 3 moles of Mg will have 6 electrons in all and 2 moles of nitrogen will have 6 electrons. So, the formula unit will be
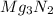 .
.
Answer 9) Option B) N
Explanation : Amongst the given choices only nitrogen will need 3 electrons to fill its valence energy shell and attain octet state. As the atomic number of nitrogen is 7, the electron distribution will be
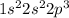 so, it is easy or nitrogen to gain 3 electrons and fulfill the valence shell and be an octet state.
so, it is easy or nitrogen to gain 3 electrons and fulfill the valence shell and be an octet state.