Answer:
The new pressure is 1,135.67 torr
Step-by-step explanation:
Boyle's law establishes the relationship between pressure and volume of a gas when the temperature is constant, establishing that the pressure of a gas in a closed container is inversely proportional to the volume of the container. Mathematically, this law says that the product of pressure and volume is constant:
P*V=k
Charles's law establishes the relationship between the temperature and the volume of a gas when the pressure is constant. This law states that the volume is directly proportional to the temperature of the gas. So, Charles's law says that the quotient that exists between the volume and the temperature will always have the same value:
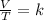
Finally, Gay-Lussac's Law is a gas law that relates pressure and temperature at constant volume. This law says that the pressure of the gas is directly proportional to its temperature:
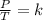
Combined law equation is the combination of three gas laws called Boyle's, Charlie's and Gay-Lusac's law:
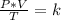
Analyzing an initial state 1 and a final state 2, it is satisfied:
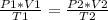
In this case:
- P1= 662 torr
- V1= 46.7 L
- T1= 266 K
- P2= ?
- V2= 35 L
- T2= 342 K
Replacing:
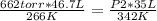
Solving:
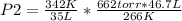
P2= 1,135.67 torr
The new pressure is 1,135.67 torr