Answer:
The correct answer is option D.
Step-by-step explanation:
In the given compound
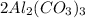
In one molecule of
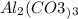 there are 9 oxygen atoms.
there are 9 oxygen atoms.
Then total number of oxygen atoms in two molecules of aluminum carbonate will be:
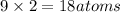
In one
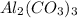 there are 9 atoms, then 2 molecule of aluminum carbonate there will be 18 atoms.
there are 9 atoms, then 2 molecule of aluminum carbonate there will be 18 atoms.