Answer: The unknown metal is Sodium.
Explanation: Photoelectric effect happens when a metal surface is hit by a monochromatic eletromagnetic wave causing the surface to emit electrons, which is the case for question.
To determine the metal, we have to find Φ or work function (W), which is the energy necessary to detach a photoelectron from the surface:
W = hf - KE
where:
h is Planck's constant and equals 6.63.
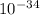 J/s;
J/s;
f is the frequency of the wave;
KE is kinetic energy the electron has the instant it detaches from the surface;
W = hf - KE
W = 6.63.
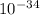 . 8.6.
. 8.6.
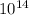 - 1.3.
- 1.3.
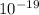
W = 57.018.
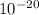 - 1.3.
- 1.3.
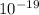
W = 4.4.
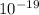 eV
eV
The element which uses this amount of energy to detach an electron is Sodium (Na).