Answer: The volume of 1.2 moles of oxygen gas (
 ) at standard temperature and pressure (STP) is 26.9 Liters
) at standard temperature and pressure (STP) is 26.9 Liters
Step-by-step explanation:
Using ideal gas equation:
PV = nRT
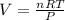
At standard conditions of temperature and pressure:
P= pressure = 1 atm
T = temperature = 273 K
V= volume = ?
Given: n = no of moles = 1.2
R= gas constant =0.0821 Latm\molK
Putting in the values we get:
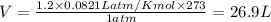
The volume of 1.2 moles of oxygen gas (
 ) at standard temperature and pressure (STP) is 26.9 Liters
) at standard temperature and pressure (STP) is 26.9 Liters