Answer: 44.8 Liters
Step-by-step explanation: According to law of conservation of mass, the sum of mass on the reactant side must be equal to the sum of mass on product side.
The balanced chemical equation is:
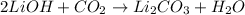
Thus 2 moles of
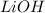 absorb 1 mole of
absorb 1 mole of
 i.e. 22.4 Liters at STP
i.e. 22.4 Liters at STP
4 moles of
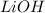 absorb
absorb
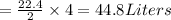
Thus 4 moles of
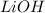 will absorb 44.8 L of
will absorb 44.8 L of
