Answer: Option (C) is the correct answer.
Step-by-step explanation:
Sucrose,
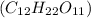 is a polar molecule as there is oxygen and hydrogen bond which will make oxygen atom partially negative and hydrogen partially positive.
is a polar molecule as there is oxygen and hydrogen bond which will make oxygen atom partially negative and hydrogen partially positive.
Also, it is known that like dissolves like. So, water being a polar molecule will dissolve sucrose.
Thus, we can conclude that the statement since water and sucrose are both polar molecules, sucrose will dissolve readily in water, BEST summarizes how sucrose will dissolve in water.