Step-by-step explanation:
The given data is as follows.
n = 0.56 moles, T =
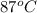 = (87 + 273) K = 360 K
= (87 + 273) K = 360 K
P = 569 torr = 0.748 atm (as 1 torr = 0.0013 atm)
According to ideal gas equation, PV = nRT
Therefore, putting given values into the above formula as follows.
PV = nRT
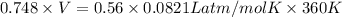
V = 22.12 L
Thus, we can conclude that the given gas will take up 22.12 L of volume.