Answer:
Energy of a single photon with the given wavelength is

Step-by-step explanation:
It is given that,
The wavelength of yellow light,
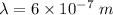
The equation that gives the relationship between the energy and the frequency of the wave is called Plank's equation. Mathematically, it can be written as :
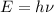
where
 is the frequency of the wave and
is the frequency of the wave and
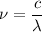
h is Planck's constant
So,
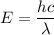
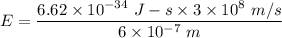

So, the energy of photon is
