Answer:
It is the time after which a given sample of radioactive substance will decay to half of the initial number present in the sample.
Step-by-step explanation:
As we know that as per law of radioactivity the number of radioactive nuclei present in the sample is proportional to the rate of decay
so it is given by
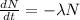
after solving above relation we will have
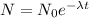
now here we know that as the number of nuclei is reduced to half of the initial number then we have
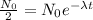
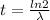
so this time is known as half life where the denominator of the equation is known as decay constant.