Answer: The correct answer is Option C.
Step-by-step explanation:
Covalent bond is formed when there is sharing of electrons between the atoms that are forming a bond.
When hydrogen and oxygen form a covalent bond to form a compound named water, both the atoms share electrons with each other.
Electronic configuration of Hydrogen =
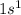
Electronic configuration of Oxygen =
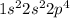
As, 2 electrons are required for one oxygen atom to complete its octet and 1 electron is required by the hydrogen to have a stable configuration. Therefore, 2 atoms of hydrogen shares its electrons to 1 atom of oxygen to form water.
The chemical formula for water is
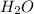 .
.
Hence, the correct answer is option C.