Electronic Configuration:
Normally, the electronic configuration will be written through the atomic number of the particular element. The electrons fill in this accruing to the energy level and orbitals like s, p, d, and f
The atomic number for the Beryllium is 4. So, the electronic configuration of beryllium is
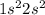
In option B, the s block has two electrons and both are having an upward spin orientation. This is incorrect, as one must be in the upward orientation and the other will be in the downward orientation