Answer: producing colored sparks in fireworks and rusting in the presence of water and oxygen
Explanation: Chemical properties are properties which involve change in chemical composition of substances.
Rusting is oxidation of iron to ferric oxide.
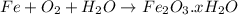
Colored sparks are produced when hot iron reacts with oxygen in the presence of oxygen.
Changing from liquid to gas at 2,862°C: It represents a physical change as there is no change in chemical composition.
Changing from solid to liquid if heated to 1,538°C: It represents a physical change as there is no change in chemical composition.
Conducting electricity and heat: It represents a physical change as there is no change in chemical composition.