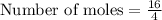Answer:
4 moles of He
Step-by-step explanation:
We have, 16g of He (Helium).
To calculate the number of moles in given amount of a substance, we have the formula.
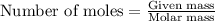 .
.
Here, we have 16g of He.
So,
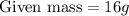
Molar mass of He is 4g
On substituting the values in the equation, we get