Answer: This will lead to the formation of ionic compound.
Step-by-step explanation:
Ionic compound is defined as the compound which is formed from the complete transfer of electrons from one atom to another atom. The atom which looses its electron is known as electropositive atom and the atoms which gains electron is known as electronegative atom.
Covalent compound is defined as the compound which is formed when sharing of electrons takes place between the atoms forming a bond.
Sodium is the 11th element of the periodic table having electronic configuration:
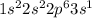
This element will loose 1 electron to attain stable electronic configuration and forms
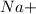 ion.
ion.
Chlorine is the 17th element of the periodic table having electronic configuration:
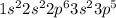
This element will gain 1 electron to attain stable electronic configuration and forms
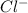 ion.
ion.
Hence, an electron is transferred from sodium to chlorine and it results in the formation of an ionic compound.
Thus, when sodium ion is attracted to chlorine ion, it leads to the formation of an ionic compound.