Answer: The correct answer is Option A.
Step-by-step explanation:
Gibbs' free energy is defined as the energy which is used to do work. It is represented as

It is also defined as the enthalpy change of the system minus the product of temperature and entropy change.
Mathematically,
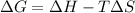
where,
 = Gibbs' free energy
= Gibbs' free energy
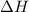 = Enthalpy change
= Enthalpy change
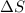 = Entropy change
= Entropy change
T = absolute temperature
When
 comes out to be negative, the reaction is spontaneous and if comes out to be positive, the reaction is non-spontaneous.
comes out to be negative, the reaction is spontaneous and if comes out to be positive, the reaction is non-spontaneous.
Hence, the correct answer is Option A.