Answer : The correct option is,
![[I_2]<[IBr]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9vymurnp7cnusa2kekpwjbjpxea8ye1335.png)
Solution : Given,
Moles of
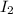 = Moles of
= Moles of
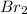
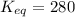
The balanced equilibrium reaction is,
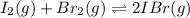
The expression for equilibrium constant is,
![K_(eq)=([IBr]^2)/([I_2][Br_2])](https://img.qammunity.org/2018/formulas/chemistry/high-school/ydgcuspbmpjwgfh7oebj08fvhfnqdxzeuq.png)
As per question, Moles of
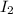 = Moles of
= Moles of
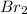
Now put all the given values in above formula, we get the relation between the
![[IBr]](https://img.qammunity.org/2018/formulas/chemistry/high-school/t2thagx7vbsn1l7fpja15iibo2s5vx3ws5.png) and
and
![[I_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/lfviq2g2le6ll84v0rrvx5lqwrlcfqfrce.png) .
.
![280=([IBr]^2)/([I_2][I_2])](https://img.qammunity.org/2018/formulas/chemistry/high-school/h7mrsdrzlnuxhj1eb9aqe27u6mho8tb3dy.png)
![280=([IBr]^2)/([I_2]^2)](https://img.qammunity.org/2018/formulas/chemistry/high-school/24ghtfbyvotsr9sbcdx7sp5y9y4f5rxdl7.png)
![√(280)=([IBr])/([I_2])](https://img.qammunity.org/2018/formulas/chemistry/high-school/w95wz06bj613izxku9qvq39pi1nfuzvinb.png)
![[IBr]=16.733* [I_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/voytsoifjgy1zawzxx19lvb96mxdlz3638.png)
From this we conclude that the concentration of
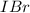 is greater than the concentration of
is greater than the concentration of
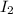 .
.
Therefore, the correct option is,
![[I_2]<[IBr]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9vymurnp7cnusa2kekpwjbjpxea8ye1335.png)