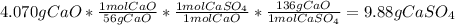Answer:
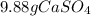
Step-by-step explanation:
1. Find the balanced equation:
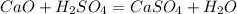
2. Find the molecular weight of the
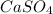
Atomic weight of Ca = 40
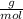
Atomic weight of S = 32
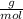
Atomic weight of O = 16
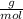
Molecular weight of
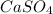 = 40 + 32 + (4*16)
= 40 + 32 + (4*16)
Molecular weight of
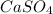 = 136
= 136
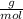
3. Use the stoichiometry to find the mass of
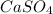 that can be produced:
that can be produced: