Answer: 5.2 moles of H2O would be produced from 5.2 moles of H2
Step-by-step explanation:
The question requires us to calculate the amount of water (H2O), in moles), that would be produced from 5.2 moles of hydrogen gas (H2), using the following balanced chemical equation:
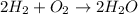
According to the balanced chemical equation, 2 moles of H2 are required to produce 2 moles of H2O. With this information, we can calculate how many moles of H2O would be produced from 5.2 moles of H2:
2 mol H2 ------------------------- 2 mol H2O
5.2 mol H2 ---------------------- x
Solving for x, we'll have:
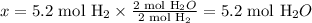
Therefore, 5.2 moles of H2O would be produced from 5.2 moles of H2.