x = amount of 55% solution in mL
y= amount of 79% solution in mL

now.. notice, we use the decimal format for the percents, thus 55% is just 55/100, and 79% is just 79/100 and 71% is well, you guessed it, 71/100 or 0.71 anyway
whatever those amounts of x and y are, they will end up as 120mL total
so, we can say that
x + y = 120
now, the concentration in each, they'll add up to (120)(0.71)
thus
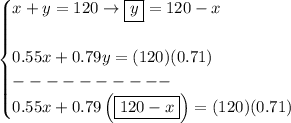
solve for "x" to see how much 55% solution will go into the 71% mix
what about the 79% solution? well y = 120 - x