ANSWER
The molar mass of carbon monoxide is 28g/mol or 2.8 x 10^1 g/mol
Step-by-step explanation
Given that
The chemical formula of carbon monoxide is CO
Note that, the unit mass of carbon is 12.01u and the unit mass of oxygen is 15.99u
Based on the atomic masses of the elements, we can now find the molar mass of CO
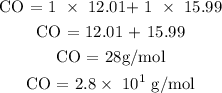
Therefore, the molar mass of carbon monoxide is 28g/mol or 2.8 x 10^1 g/mol