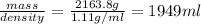Answer:
Around 2.0 L of ethylene glycol needs to be added to the car radiator
Step-by-step explanation:
The depression in freezing point ΔTf of a solution is directly proportional to its molality (m), i.e.
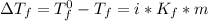
From the given information:
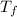 = freezing pt of solution = -10.0 C
= freezing pt of solution = -10.0 C
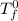 = freezing pt of pure solvent = 0 C
= freezing pt of pure solvent = 0 C
Kf = freezing pt depression constant = 1.86 C/m
i = 1 for ethylene glycol antifreeze
![[0-(-10.0)] C= 1*(1.86 C/m) *( m)\\\\m = 5.38](https://img.qammunity.org/2018/formulas/chemistry/college/tbfif3rcxi70l0mu6v4ph6oq04mz3vyc7t.png)
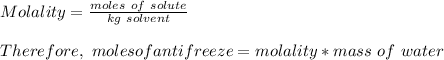
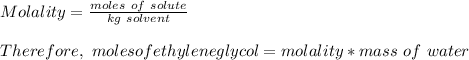
Volume of water = 6.50 L = 6500 ml
Density of water = 1.00 g/ml
Therefore mass of water =
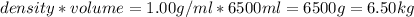
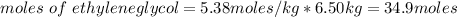
Molar mass of ethylene glycol = 62 g/mol
Mass of ethylene glycol needed =
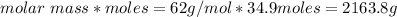
Density of ethylene = 1.11 g/ml
Therefore, volume needed =