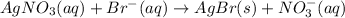Answer: A)
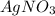
Explanation: All acetates are soluble.
All compounds of Alkali metals are soluble.
Bromides of Barium, and copper are soluble but that of silver is insoluble.
So, to separate bromide ion from acetate ion, we need to mix silver nitrate to the mixture.
Hence, the right choice is A)
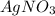 .
.