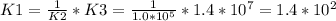Answer:
K1 = 1.4*10^2
Step-by-step explanation:
The given reactions are:
Reaction 1
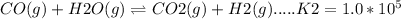
Reaction 2
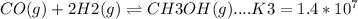
The required reaction is:
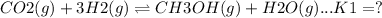
This reaction can be obtained by reversing the first reaction and then adding the reactions 1 and 2.
As per convention
1) For a multistep reaction, the net equilibrium constant is the product of Keq of the individual steps
2) if a reaction is reversed the new equilibrium constant will be the inverse of the old equilibrium constant.
Therefore,