Answer : The correct option is, (A) 51.1%
Explanation :
Mass percent : It is defined as the mass of the given component present in the total mass of the compound.
Formula used :
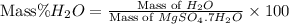
First we have to calculate the mass of
 and
and
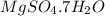 .
.
Mass of
 = 18 g/mole
= 18 g/mole
Mass of
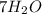 = 7 × 18 g/mole = 126 g/mole
= 7 × 18 g/mole = 126 g/mole
Mass of
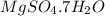 = 246.47 g/mole
= 246.47 g/mole
Now put all the given values in the above formula, we get the mass percent of
 in
in
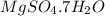 .
.
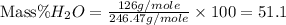
Therefore, the mass percent of
 in
in
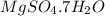 is, 51.1%
is, 51.1%