The mass number corresponds to the sum of the number of protons plus neutrons. When the number of neutrons differs from the number of protons, we have an isotope, the same element but with a different mass number.
Iron has an atomic number equal to 26, which means that it has 26 protons. If the iron atom has 24 neutrons its mass number will be:
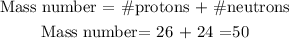
The mass number of iron with 24 neutrons is 50