Answer: 24 grams of element would remain.
Explanation:
Exponential function for decay:
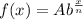 ....(i) , where A = initial value , b = decay factor, x = time , n= time per period
....(i) , where A = initial value , b = decay factor, x = time , n= time per period
As per given,
A = 569 grams ,
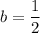
n= 5 years, t = 23 years
Put all values in (i) , we get

Hence, after 23 years , 24 grams of element would remain.