Answer:
B and C
Explanation:
We are given that
A.
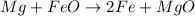
On left side
Number of atoms of Fe =1
Number of atoms of O=1
Number of atoms of Mg=1
On right side
Number of atoms of Fe=2
Number of atoms of O=1
Number of atoms of Mg=1
Therefore ,the balance reaction is
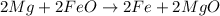
Given reaction is not balanced reaction
B.
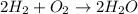
Number of atoms of oxygen and hydrogen are equal on both sides .
Hence, the given reaction is balanced .
C.
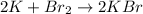
The number of atoms of potassium and bromine are equal on both sides.
Therefore, the given reaction is balanced .
D.
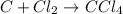
Number of atoms of Chlorine are not equal on both sides therefore, the given reaction is not balanced.
Hence,option B and C are true.