Answer:
Maximum kinetic energy,
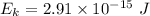
Step-by-step explanation:
It is given that,
Speed of photoelectrons,
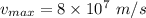
Mass of electrons,
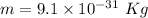
Frequency of light,
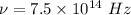
According to Einstein equation :
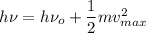
Where
υ is frequency of radiation
υ₀ is the threshold frequency
Maximum kinetic energy of single electron is,
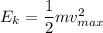
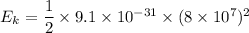
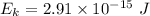
Hence, the correct option is (C) "
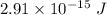 "
"