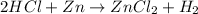Answer: Option (a) is the correct answer.
Step-by-step explanation:
The given equation is as follows.
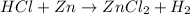
Number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
To balance the equation, multiply HCl by 2 on reactant side. Therefore, the balanced chemical equation is as follows.