Answer:
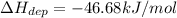
Step-by-step explanation:
Hello!
In this case, since vaporization is the processes by which a liquid goes to the gas phase, fusion by which a solid goes to liquid and deposition by which a gas goes to solid; we infer that the following set up relates the enthalpies associated to each process:
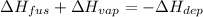
Because deposition goes from a state with more energy to a state with less energy, therefore it is negative; in such a way, by plugging in we obtain:
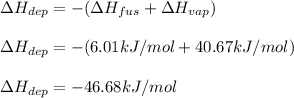
Best regards!