You can work out the concentration of H+ ions (denoted as [H+]) easily on a calculator if you know the pH, which you do. The easiest way to work out [H+] is to do
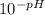
. In your case, the maths here would be
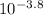
, which gets you an answer of 1.58x
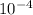
, or 0.000158. For 6.2 it would be
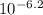
, which gives you 6.31x
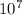
, or 0.00000063. As you can see, the concentration of H+ ions is greater in pH 3.8.
To put it simply, the [H+] decreases the higher up the pH scale you go, and increases as you go lower, but now you know the maths to work it out for yourself too.